(Shanghai, China, June 23, 2020) - CStone Pharmaceuticals Co., Ltd. (“CStone” or the “Company”, HKEX: 2616) today announced that the company released the pre-clinical data of its three pipeline products, i.e. CS1001 (anti-PD-L1 monoclonal antibody), CS3002 (CDK4/6 selective small molecule inhibitor) and CS3003 (HDAC6 selective small molecule inhibitor), in the E-poster presentation session at the 2020 AACR Virtual Annual Meeting II. In addition, the company recently (May 28, 2020) published a peer-reviewed article in Acta Pharmacologica Sinica, in which the preclinical characterization of CS1003 (anti-PD-1 monoclonal antibody) was fully illustrated.
“Since its establishment at the end of 2015, CStone has successfully advanced 7 drug candidates, each developed independently by CStone, into the clinical stage. The three posters recently published at the AACR Annual Meeting II, and the peer-reviewed article published in Acta Pharmacologica Sinica, have shown the differentiated advantages of the four core assets, including two backbone IO therapies CS1001 (PD-L1 monocolonal antibody) and CS1003 (PD-1 monocolonal antibody), a CDK4/6 small molecule inhibitor for cell cycle regulation and an HDAC6 small molecule inhibitor for epigenetic regulation, both of which have shown great therapeutic potential in combination with immune checkpoint inhibitors. We will formulate a more competitive clinical development strategy based on a deep understanding of the drugs’ mechanism of action. Leveraging our strong internal research & development(R&D)capabilities, we will also promote the company's transition towards Pipeline 2.0, in order to provide an impetus for the continued stable development of the company in the future”, Dr. Jon Wang, Chief Scientific Officer of CStone Pharmaceuticals said.
CS1001(PD-L1)
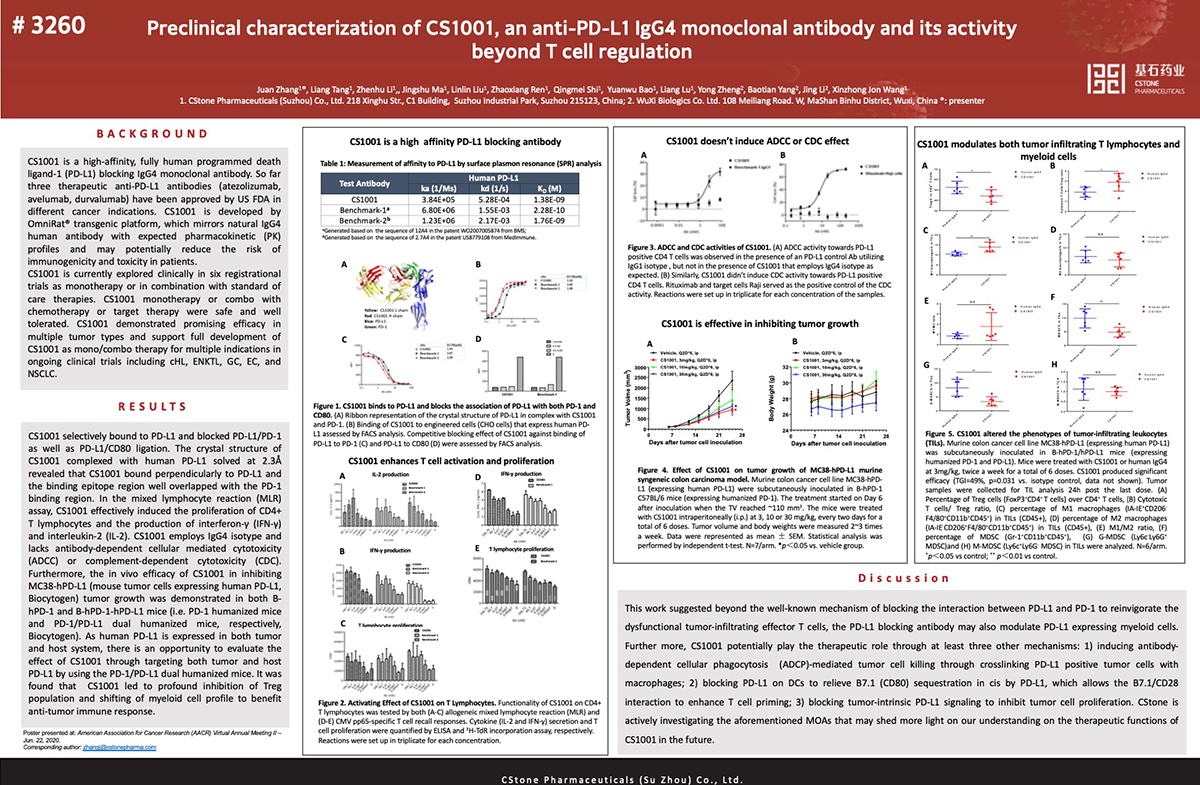
CS1001 is an investigational anti-PD-L1 monoclonal antibody being developed by CStone. It can inhibit tumor growth by blocking the interactions between PD-L1 with PD-1. The data for CS1001, which was previously presented at the annual meetings of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the American Society of Hematology (ASH), and the Chinese Society of Clinical Oncology (CSCO), show that CS1001 has demonstrated promising efficacy and safety in a variety of solid tumors and lymphomas.
The pre-clinical data presented at the 2020 AACR Virtual Annual Meeting II revealed that in addition to reinvigorating the dysfunctional T cells by blockade of the PD-1/PD-L1 interaction, CS1001 can also alter the myeloid cell profile within tumor microenvironment (TME) to benefit anti-tumor immune responses.
E-poster Title: Preclinical characterization of CS1001, an anti-PD-L1 IgG4 monoclonal antibody, and its activity beyond T cell regulation(Abstract # 3260)
CS3002
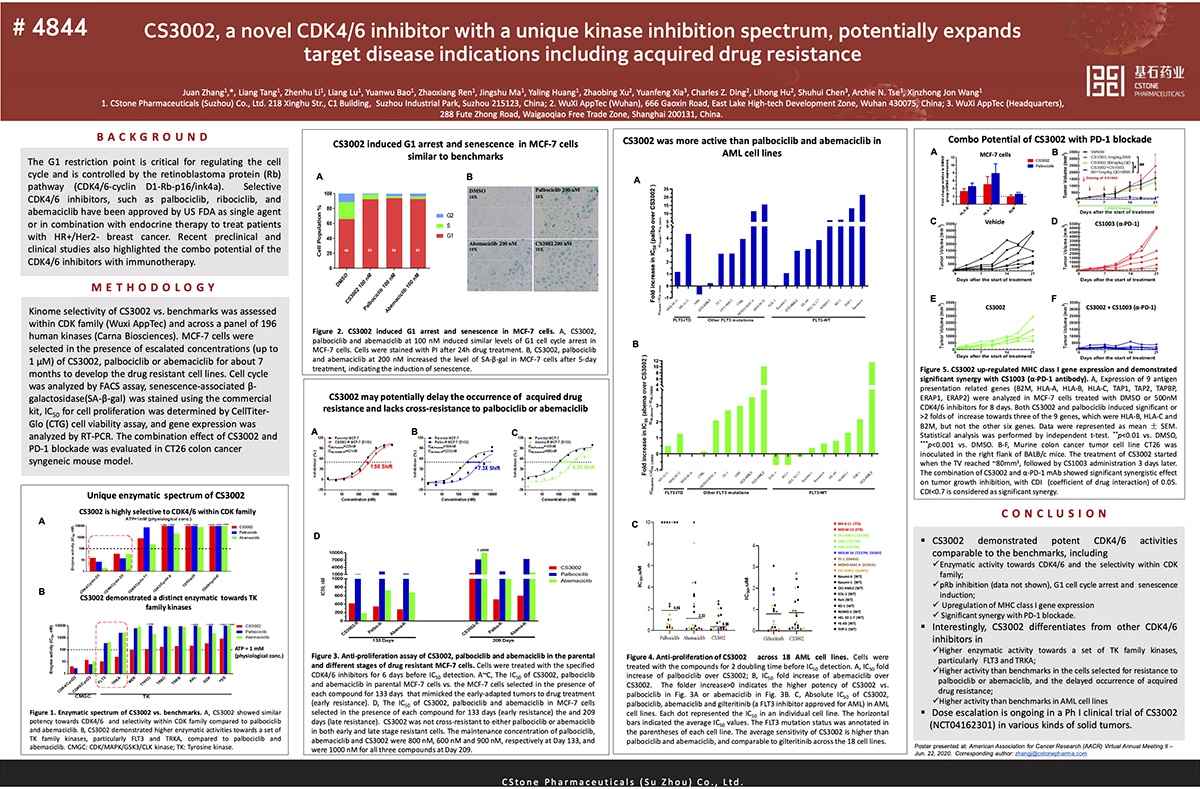
CS3002 is a selective small molecule inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6) being developed by CStone Pharmaceuticals. CS3002 induces cell cycle arrest of tumor cells through direct inhibition of CDK4/6 kinase activities. Currently, only one CDK4/6 inhibitor—palbociclib—has been approved in China. Preclinical studies have shown that the in vivo and in vitro activities of CS3002 are comparable to those of palbociclib. In mouse models, CS3002 in combination with anti-PD-1 monoclonal antibody therapy or endocrine therapy has shown improved anti-tumor activities compared to mono-therapies. CS3002 Phase I study has started in Australia, and its IND application has also been approved in China. These trials are designed to evaluate the safety, tolerability, pharmacokinetics, and anti-tumor activity of CS3002 in patients with advanced solid tumors.
The preclinical data presented at the 2020 AACR Annual Meeting II demonstrated the anti-tumor effect of CS3002 in cells selected for resistance to approved CDK4/6 inhibitors, as well as its immunomodulatory functions, indicating CS3002 has potential application in drug-resistant tumor cells and IO combination therapies.
E-poster Title: CS3002, a novel CDK4/6 inhibitor with a unique kinase inhibition spectrum, potentially expands target disease indications including acquired drug resistance(Abstract #:4844)
CS3003
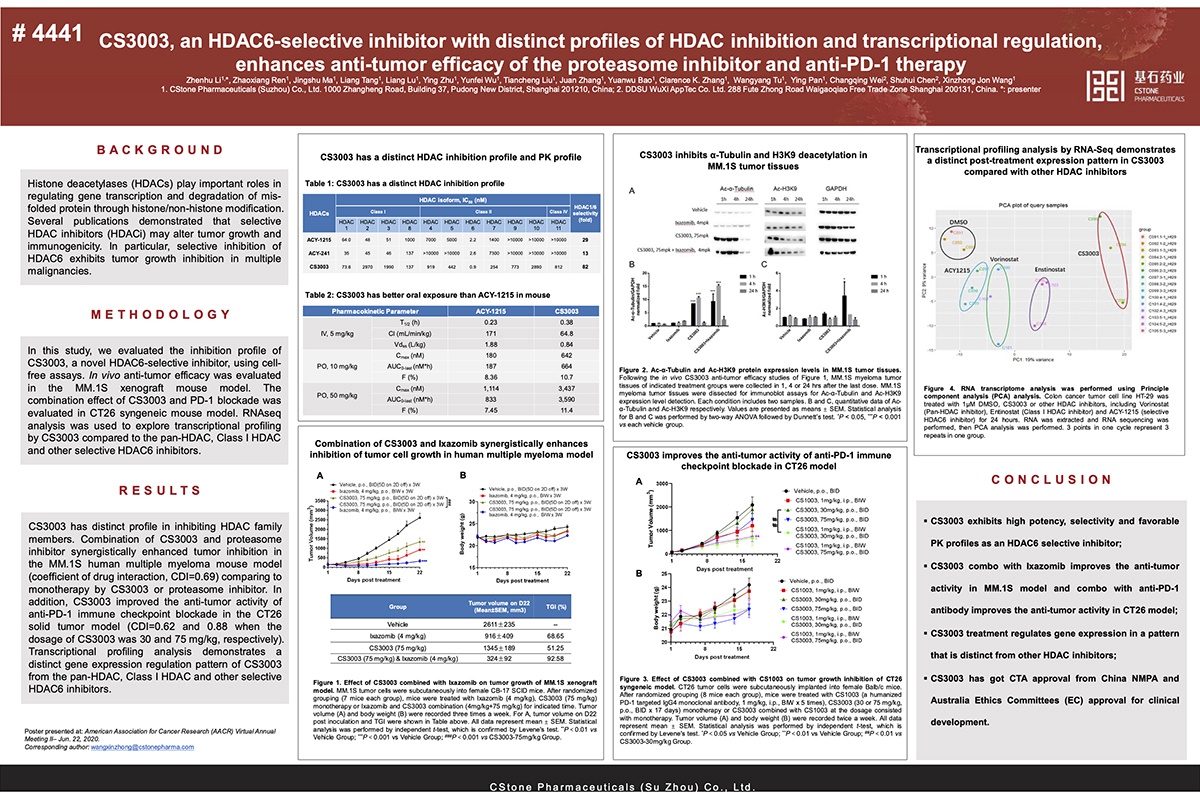
CS3003 is a small molecule inhibitor that selectively targets histone deacetylase 6 (HDAC6). By inhibiting HDAC6-mediated degradation of misfolded proteins and increasing cellular stress, it induces tumor cell apoptosis to achieve anti-tumor effects. Previous preclinical studies have shown that the combined use of HDAC6 selective inhibitors and proteasome inhibitors in multiple myelomas resulted in better efficacy and improved safety over pan-HDAC inhibitors. At present, the Phase I clinical trial to evaluate the safety and efficacy of CS3003 in advanced solid tumors and relapsed / refractory multiple myeloma has been approved in both China and Australia.
The pre-clinical data presented at the 2020 AACR Virtual Annual Meeting II demonstrated the improved efficacy of CS3003 in combination with proteasome inhibitors and immune checkpoint inhibitors over mono-therapies, and revealed its unique modulation of gene expression as compared to other HDAC inhibitors.
E-poster Title: CS3003, an HDAC6-selective inhibitor with distinct profile of HDAC inhibition and transcriptional regulation, enhances anti-tumor efficacy of proteasome inhibitor and anti-PD-1 therapy (Abstract #: 4441)
CS1003
CS1003 is a humanized IgG4 PD-1 monoclonal antibody that can specifically bind to human, monkey and mouse PD-1. It is designed to block the interaction between PD-1 and its ligands PD-L1 and PD-L2 for the immunotherapy in multiple tumor types. The Phase I clinical trial of CS1003 is currently being conducted in Australia, New Zealand and China. In addition, patient enrollment of a global multi-center Phase III study to evaluate CS1003 in combination with Lenvima (lenvatinib) as the first-line treatment for advanced HCC is on-going.
CS1003, a novel human and mouse cross-reactive PD-1 monoclonal antibody for cancer treatment
The article published in Acta Pharmacologica Sinica shows that CS1003 has high affinity to human, mouse and monkey PD1, and can inhibit the interaction between PD-1 and its ligands PD-L1 and PD-L2, thereby blocking PD- 1 mediated immunosuppressive activity, which in turn enhances T cell cytokine production and promotes T cell proliferation. In MC38mouse syngeneic colon cancer model and MC38-h-PD-L1 colon cancer engrafted in hPD-1 knock-in mouse model, CS1003 has exhibited good antitumor activity in vivo. The ability of CS1003 to cross-react to both human and mouse PD-1 has allowed a convenient evaluation on the combination potential of CS1003 with other therapeutics in animal models. CStone has conducted several studies to investigate the therapeutic potential of CS1003 in combination with CStone's other pipeline products or other innovative drugs. The pharmacokinetic (PK) study in cynomolgus monkeys following single intravenous administration showed a dose-proportional increase in exposure through the dose range of 2-18 mg/kg. In toxicity study in cynomolgus monkeys, CS1003 has demonstrated a favorable safety profile with the highest non-severely toxic dose (HNSTD) at 100mg/kg.
Li F, Li J, Yin K, et al. CS1003, a novel human and mouse cross-reactive PD-1 monoclonal antibody for cancer therapy. Acta Pharmacol Sin. 2020;10.1038/s41401-020-0422-6. doi:10.1038/s41401-020-0422-6
About CS1001
CS1001 is an investigational monoclonal antibody directed against PD-L1, currently being developed by CStone. Authorized by Ligand Pharmaceuticals Incorporated (LGND, U.S.), CS1001 is developed by the OmniRat® transgenic animal platform, which can generate fully human antibodies in one step. As a fully human, full-length anti-PD-L1 monoclonal antibody, CS1001 mirrors natural G-type immunoglobulin 4 (IgG4) human antibody, which can reduce the risk of immunogenicity and potential toxicities in patients, potentially representing a unique advantage over similar drugs.
CS1001 has completed a Phase I dose-escalation study in China, in which it demonstrated good tolerability and produced sustained clinical benefits during the Phase Ia and Ib stages of the study.
CS1001 is being investigated in a number of ongoing clinical trials, including one Phase I bridging study in the U.S., one multi-arm Phase Ib study, two Phase II registrational studies, and four Phase III studies in patients with stage III & IV NSCLC, gastric cancer and esophageal cancer.
About CS3002
CS3002 is a new generation of well-tolerated and highly selective CDK4/6 inhibitor developed by CStone.
CDK4/6 inhibitors are the cyclin-dependent kinases that play a crucial role in the regulation of cell cycle progression from the Gap 1 phase (G1 phase) to the Synthesis phase (S phase). Upon activation of the cell proliferation signal, cyclin D protein binds to CDK4/6. The cyclin D–CDK4/6 complex then phosphorylates downstream retinoblastoma (Rb) protein, resulting in the aberrant proliferative signaling in the CDK4/6 pathway that drives the cell cycle progression from the G1 phase to the S phase. Aberrant CDK activity is a common feature of most cancer types. CDK4/6 inhibitors could suppress the activities of CDK4/6 and the phosphorylation of Rb protein, thereby achieving the suppression of tumor cell growth by interrupting the cell cycle transition from the G1 phase to the S phase. The fact that dysregulation of the cyclin D-CDK4/6-INK4-Rb is frequently observed in many types of cancer and that CDK4/6 inhibitors have the potential to augment anti-tumor immunity indicates CDK4/6 inhibitors have broad application when used in the treatment of various solid tumors and in immune-oncology combination therapies.
About CS3003
CS3003 is a small molecule inhibitor that selectively targets histone deacetylase 6 (HDAC6). Unlike the other HDAC family members, HDAC6 is mainly located in the cytoplasm and has little effect on DNA histone acetylation. HDAC6 inhibition can enhance acetylation of cytoplasmic tubulin and lose the capability to clear unfolded or misfolded proteins, thereby promoting cell apoptosis. Selective inhibition of HDAC6 produces better efficacy in multiple myeloma and has improved safety profile over pan-HDAC inhibitors. CS3003 also has the potential to combine with PD-(L)1 antibody drugs in solid tumors to expand the clinical efficacy of immune checkpoint inhibitors.
About CS1003
PD-1 (Programmed cell death protein 1) is an inhibitory receptor that is preferentially expressed in T cells. Under normal physiological conditions, PD-1 will bind to programmed death ligand 1 or ligand 2 (PD-L1 / PD-L2), leading to reduced activity of T cells and the production of cytokines, which in turn protects the body from attacks by its own immune system. However, studies have found high level of PD-L1 expression on the surface of many solid tumor and some hematological malignant tumor cells in human. Tumor cells can successfully escape the identification and attack of the body's immune system through the binding of PD-L1 with PD-1 on T cells. Anti-cancer drugs such as PD-1 / PD-L1 immune checkpoint inhibitors can block this “tumor immune escape mechanism" and restore the anti-cancer function by the patients’ immune system.
CS1003 is a humanized recombinant IgG4 monoclonal antibody targeting human programmed cell death protein 1 (PD-1), which is being developed for immunotherapy of various tumors. Compared to most of the anti-PD-1 monoclonal antibodies that bind to human and monkey PD-1 (those that have either been approved or are being investigated in clinical trials), CS1003 can bind to both human and murine PD-1, and has shown unique competitive advantages in synergistic mouse models for drug efficacy testing.
Currently, the phase I clinical trial of CS1003 is being conducted in Australia, New Zealand and China. In addition, patient enrollment of a global multi-center Phase III study to evaluate CS1003 in combination with Lenvima (lenvatinib) as the first-line treatment for advanced HCC is on-going.
About CStone
CStone Pharmaceuticals (HKEX:2616) is a biopharmaceutical company focused on developing and commercializing innovative immuno-oncology and precision medicines to address the unmet medical needs of cancer patients in China and worldwide. Established in 2015, CStone has assembled a world-class management team with extensive experience in innovative drug development, clinical research, and commercialization. The company has built an oncology-focused pipeline of 15 drug candidates with a strategic emphasis on immuno-oncology combination therapies. Currently, five late-stage candidates are at or near pivotal trials. With an experienced team, a rich pipeline, a robust clinical development-driven business model and substantial funding, CStone’s vision is to become globally recognized as a leading Chinese biopharmaceutical company by bringing innovative oncology therapies to cancer patients worldwide.
For more information about CStone Pharmaceuticals, please visit: www.cstonepharma.com
Forward-looking Statement
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
Your privacy is important for us. We use cookies to enhance your experience when visiting our websites: performance cookies show us how you use this website, functional cookies remember your preferences and targeting cookies help us to share content relevant to you. Select “Accept all” for giving your consent to all cookies or select “Reject all” for using only strictly necessary cookies.