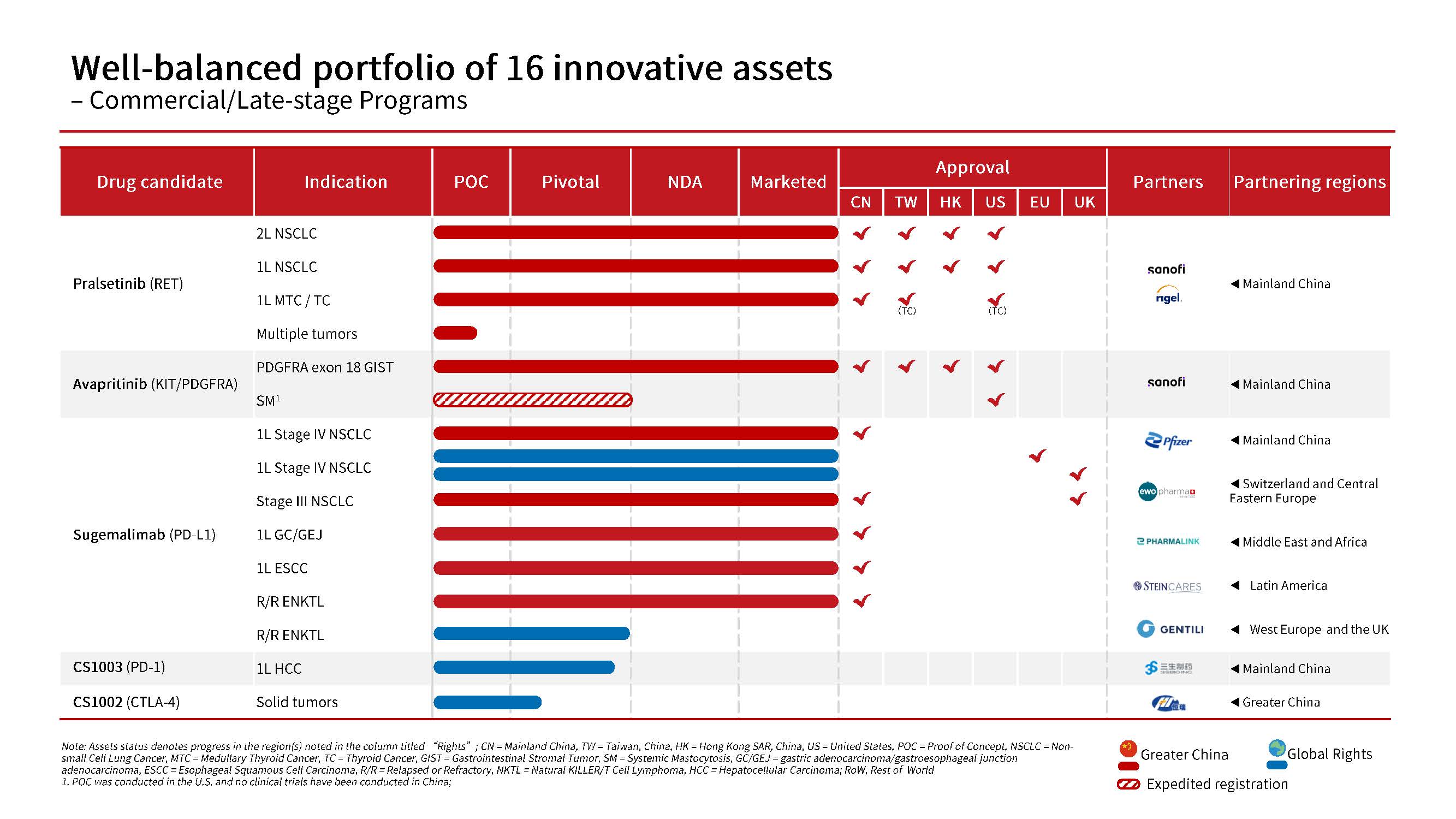
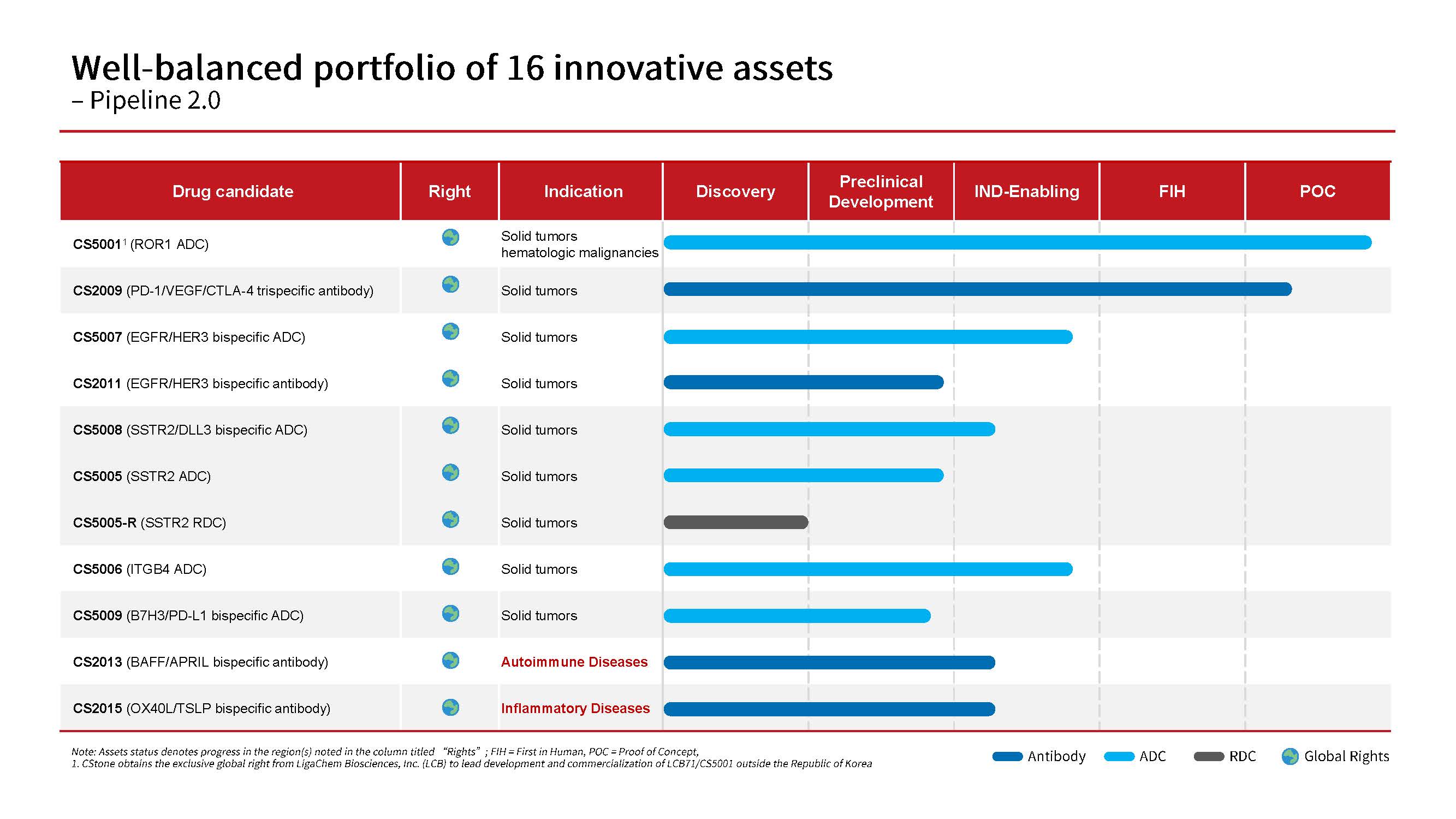
To date, the Company has successfully launched 4 innovative drugs and secured approvals for 16 new drug applications (NDAs) covering 9 indications. The company’s pipeline is balanced by 16 promising candidates, featuring potentially first-in-class or best-in-class antibody-drug conjugates (ADCs), multispecific antibodies, immunotherapies and precision medicines.
Your privacy is important for us. We use cookies to enhance your experience when visiting our websites: performance cookies show us how you use this website, functional cookies remember your preferences and targeting cookies help us to share content relevant to you. Select “Accept all” for giving your consent to all cookies or select “Reject all” for using only strictly necessary cookies.