(Suzhou, China, September 25, 2020) CStone Pharmaceuticals (Suzhou) Co., Ltd. (“CStone", HKSE: 2616) presented the results from two Phase 1 studies of CS1003 at the 2020 ESMO Meeting held from September 19th to September 21st, 2020. CS1003-101 study (Poster ID 1057P) was designed to evaluate the safety, PK, immunogenicity and preliminary anti-tumor activity of CS1003 in patients with advanced cancer, and CS1003-102 (Poster ID: 987P) was designed to evaluate CS1003 in combination with lenvatinib (LEN) as a first-line (1L) treatment for Chinese patients with unresectable hepatocellular carcinoma (uHCC). In both studies, CS1003, used either as monotherapy or in combination with standard of care (SoC), was safe and well tolerated, and demonstrated promising anti-tumor activity, supporting future clinical development of this molecule as an immune-oncology backbone agent.
CS1003 is a full-length, humanized immunoglobulin G4 (IgG4) monoclonal antibody against PD-1 developed by CStone Pharmaceuticals. Unlike other anti-PD-1 antibodies, CS1003 has comparable binding affinity against both human and mouse PD-1, which allows rapid evaluation of anti-tumor effect in syneneic mouse tumor models, including the combination with lenvatinib described herein.
About CS1003-101(NCT03475251)
The phase 1b part of the first-in-human study of CS1003 (NCT 03475251) aimed to evaluate the efficacy, safety and pharmacokinetics of two dosing schedules of CS1003, at 200 mg Q3W and at 400 mg Q6W. The primary endpoint was objective response rate (ORR) per RECIST V1.1 by investigators, and secondary endpoints included progression-free survival (PFS), disease control rate (DCR), duration of response (DOR), overall survival (OS), safety, tolerability, pharmacokinetics, and immunogenicity.
In patients with advanced solid tumors, the average plasma concentration at steady state ( Cavg,ss ) of 400mg Q6W was comparable to that of 200 mg Q3W. Both dosing regimens are well tolerated and showed promising antitumor activity with an ORR of above 20%.
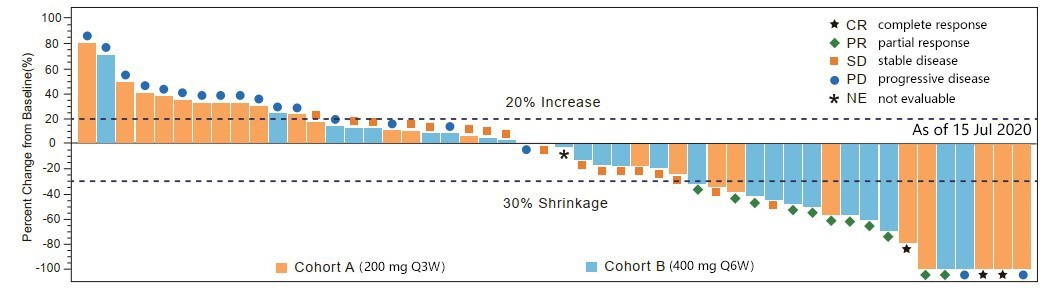
Waterfall of Maxium Target Lesion Shrinkage per RECIST v1.1 (Efficacy Analysis Set)
About CS1003-102(NCT03809767)
CS1003-102(NCT03809767) is a multi-center, open-label phase I dose escalation, and indication expansion study conducted in China , Arm 5 of Phase Ib part of which aimed to evaluate the safety and efficacy of CS1003 in combination with lenvatinib (LEN) as a first-line treatment for Chinese patients with unresectable HCC (uHCC). The primary endpoint was objective response rate (ORR) per RECIST V1.1 by investigators, and secondary endpoints included progression-free survival (PFS), disease control rate (DCR), duration of response (DOR) , overall survival (OS), safety and tolerability, pharmacokinetics, and immunogenicity.
As of June 22nd, 2020, a total of 20 patients were enrolled and received treatment. The majority of patients were male (90%), had a ECOG score of 1 (75%), had BCLC stage C HCC (90%), and had HBV infection (65%)
CS1003 in combination with lenvatinib (LEN) as a first-line treatment of Chinese patients with unresectable hepatocellular carcinoma (uHCC) demonstrated promising efficacy and was well tolerated:
Time on Treatment, Individual Best Response (Efficacy Analysis Set)
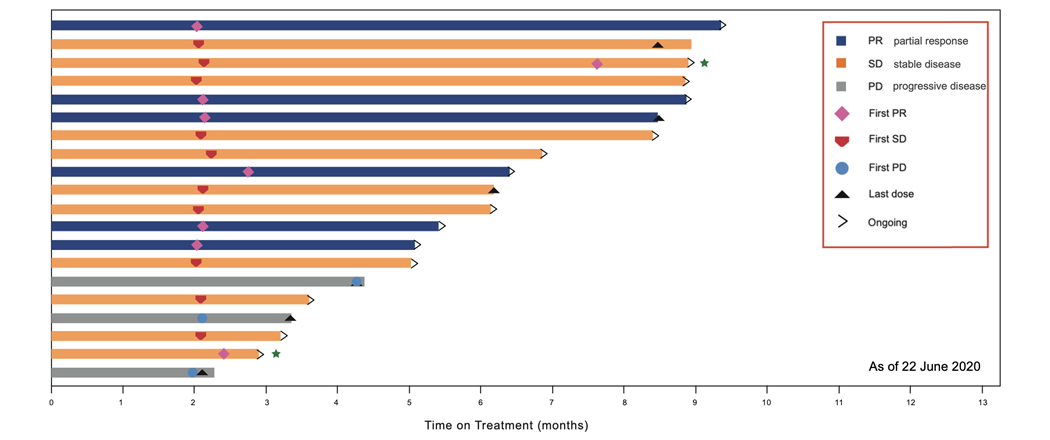
Dr. Archie Tse, Chief Translational Meidicne Officer of CStone, said, “We are very pleased to see the excellent efficacy and safety demonstrated by CS1003 monotherapy alone or in combination with standard treatment so far. CS1003 is a monoclonal antibody with high-affinity to disrupt the interaction of PD-1 with its ligands. CS1003 has demonstrated good safety and anti-tumor activity in patients with advanced solid tumors. The 400mg Q6W dosing regimen offers a more flexible and convenient dosing option for patients. In addition, as a novel anti-PD-1, CS1003 in combination with lenvatinib has resulted in an ORR of 40%, indicating a potential clinical advantage for treating advanced uHCC. All of these preliminary results support further development of CS1003 as an immune-oncology backbone agent”.
About CS1003
CS1003 is a humanized recombinant IgG4 monoclonal antibody targeting human programmed cell death protein 1 (PD-1) being developed for immunotherapy of various tumors. Compared to most of the monoclonal antibodies that bind human and monkey PD-1(either already approved or in clinical stage) , CS1003 demonstrates comparable high binding affinities across species against human, cynomolgus moneky and mouse PD-1, and is developed to disrupt the interaction of PD-1 with its liagands PD-L1 and PD-L2 . CS1003 is also unique in that it can simultaneously recognize human and mouse PD-1, which allows fast pre-clinical proof of concept for CS1003 in combination with novel targeted therapies using syngeneic mouse tumor models.
About CStone
CStone Pharmaceuticals (HKEX:2616) is a biopharmaceutical company focused on developing and commercializing innovative immuno-oncology and precision medicines to address the unmet medical needs of cancer patients in China and worldwide. Established in 2015, CStone has assembled a world-class management team with extensive experience in innovative drug development, clinical research, and commercialization. The company has built an oncology-focused pipeline of 15 drug candidates with a strategic emphasis on immuno-oncology combination therapies. Currently, five late-stage candidates are at or near pivotal trials. With an experienced team, a rich pipeline, a robust clinical development-driven business model and substantial funding, CStone’s vision is to become globally recognized as a leading Chinese biopharmaceutical company by bringing innovative oncology therapies to cancer patients worldwide.
Forward-Looking Statement
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
Your privacy is important for us. We use cookies to enhance your experience when visiting our websites: performance cookies show us how you use this website, functional cookies remember your preferences and targeting cookies help us to share content relevant to you. Select “Accept all” for giving your consent to all cookies or select “Reject all” for using only strictly necessary cookies.