Suzhou, China, November 11, 2024--CStone Pharmaceuticals (“CStone”, HKEX: 2616), an innovation-driven biopharmaceutical company focused on the research and development of anti-cancer therapies, today announced the presentation of preclinical data on CS2009, a leading asset from the Company’s Pipeline 2.0, at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC Annual Meeting). CS2009 is a trispecific antibody that simultaneously targets PD-1, CTLA-4, and VEGFA.
Key Highlight
Dr. Jason Yang, CEO, President of R&D, and Executive Director at CStone, commented, “We are excited to present the latest preclinical data on CS2009 at SITC Annual Meeting, marking its debut on the international stage. As a pivotal asset in CStone’s Pipeline 2.0, CS2009 holds the potential to become a next-generation, first- or best-in-class immunotherapy backbone to replace the current anti-PD-(L)1 therapies. These compelling preclinical results strengthen our confidence in advancing its clinical development. We look forward to seeing CS2009 to benefit patients with various cancers, including non-small cell lung cancer, ovarian cancer, renal cell carcinoma, cervical cancer, hepatocellular carcinoma, and gastric cancer, particularly those with low or negative PD-L1 expression who respond poorly to PD-(L)1 therapies.”
Key Findings
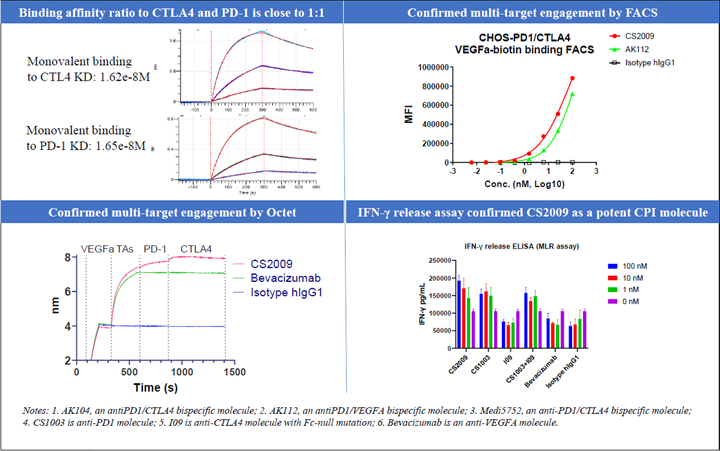
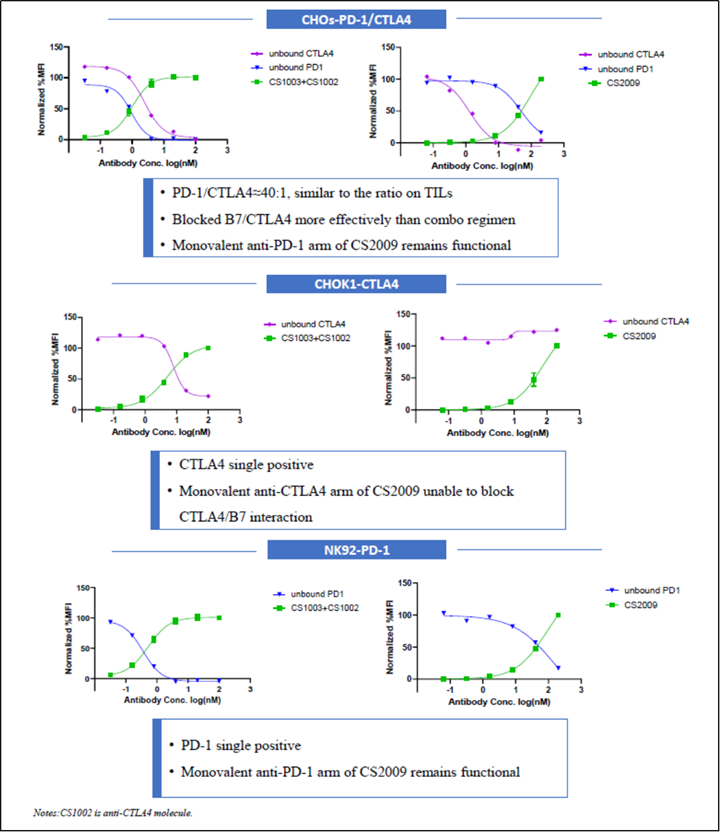
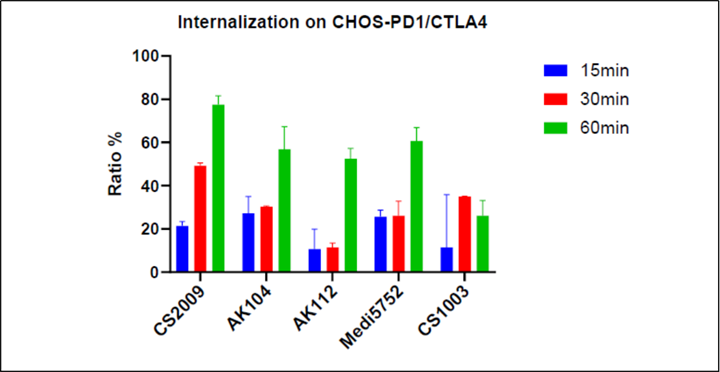
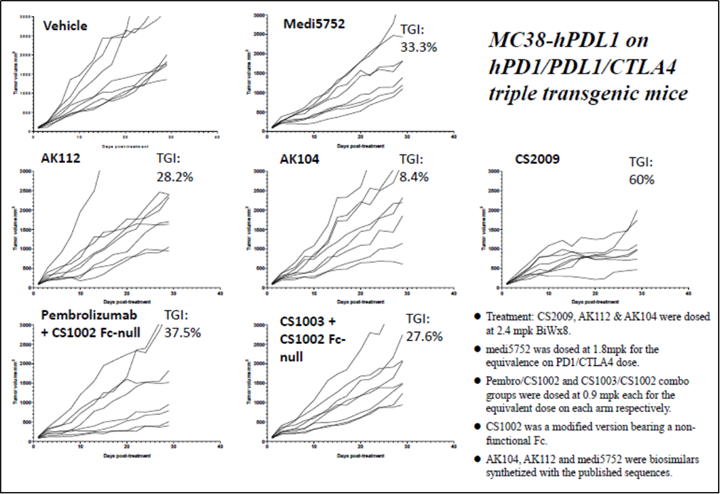
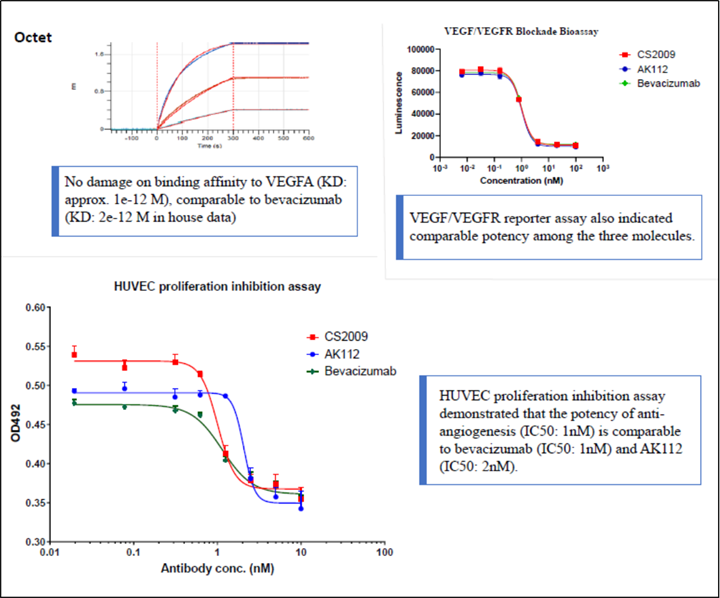
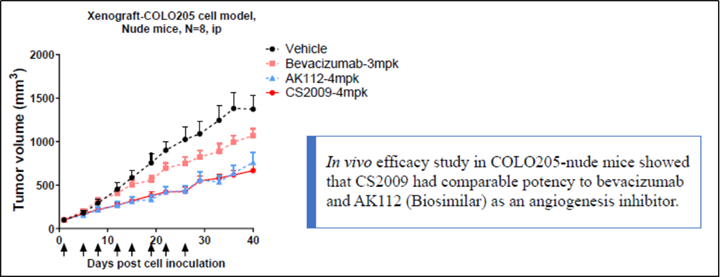
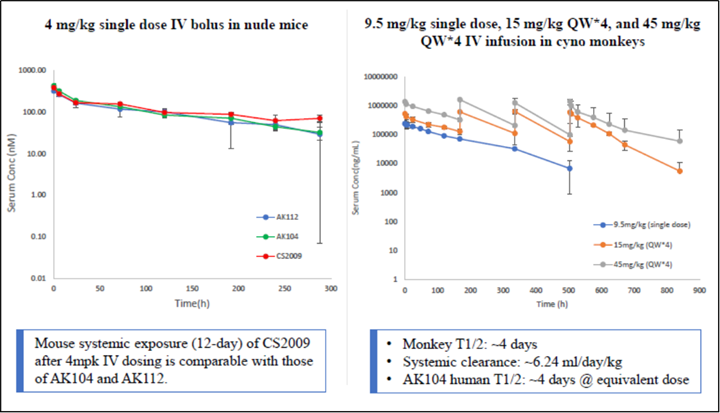
CS2009 is currently in preparation for an IND submission, with filing anticipated by late 2024 or early 2025 and first-in-human trial to be initiated in early 2025.
About CS2009 (PD-1/CTLA4/VEGFA Trispecific Antibody)
CS2009 is a trispecific antibody targeting PD-1, CTLA-4, and VEGFA, with the potential to be first- or best-in-class for major tumor types. CS2009 has a differentiated molecular design that combines three clinically validated targets, preferentially invigorating exhausted TILs and demonstrating VEGFA neutralization comparable to existing anti-VEGFA antibodies. It covers a wide range of cancers, including non-small cell lung cancer, ovarian cancer, renal cell carcinoma, cervical cancer, hepatocellular carcinoma, and gastric cancer.
About CStone
CStone (HKEX: 2616), established in late 2015, is an innovation-driven biopharmaceutical company focused on the research and development of anti-cancer therapies. Dedicated to addressing patients’ unmet medical needs in China and globally, the Company has made significant strides since its inception. To date, the Company has successfully launched 4 innovative drugs and secured approvals for 16 new drug applications (NDAs) covering 9 indications. The Company’s pipeline is balanced by 18 promising candidates, featuring potentially first-in-class or best-in-class antibody-drug conjugates (ADCs), multispecific antibodies, immunotherapies and precision medicines. CStone also prides itself on a management team with comprehensive experiences and capabilities that span the entire drug development spectrum, from preclinical and translational research to clinical development, drug manufacturing, business development, and commercialization.
For more information about CStone, please visit: www.cstonepharma.com.
IR contact: ir@cstonepharma.com
PR contact: pr@cstonepharma.com
Forward-looking statements
The forward-looking statements made in this article only relate to events or information as of the date when the statements are made in this article. Except as required by law, we undertake no obligation to update or publicly revise any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. All statements in this article are made on the date of publication of this article and may change due to future developments.
Disclaimer: only for communication and scientific use by medical and health professionals.
Your privacy is important for us. We use cookies to enhance your experience when visiting our websites: performance cookies show us how you use this website, functional cookies remember your preferences and targeting cookies help us to share content relevant to you. Select “Accept all” for giving your consent to all cookies or select “Reject all” for using only strictly necessary cookies.